Published online: November 2021
Synthesis by: Erica R Hendrikse, Andrew Veale, Brian Hopkins; Manaaki Whenua - Landcare Research (HendrikseE @ landcareresearch.co.nz)

Stoat with a tūī. Photo: Andrew Veale
The development of RNA-based vaccines against the virus causing COVID-19 has highlighted the use of RNA as a tool in solving complex biological problems. One major conservation issue facing Aotearoa is the eradication of mammalian predators (possums, mustelids, and rats) by 2050. The Predator Free 2050 target is challenging as current tools have limitations. No trap is efficient enough to remove 100% of individuals, and our currently available toxins are broad-spectrum; therefore, there are risks to non-target species, and social acceptability issues. The development and application of new vertebrate pest control tools is therefore critical to help us achieve our conservation goals. Although RNAi is still in the exploratory, proof-of-concept stages for vertebrate pest control, this article explores the possibility of leveraging the cellular processes of RNA interference (RNAi) for the development of a new generation of control tools that are pest-specific.
RNAi is a mechanism that regulates protein production inside most cells (illustrated in Figure 1A). RNAi is triggered by the presence of foreign double stranded RNA (dsRNA) within the cell (Figure 1B). Once activated, RNAi proteins identify any messenger RNA with the matching sequence to the dsRNA. The RNAi proteins intercept this matching messenger RNA and degrade it, disrupting the protein production for the specific protein indicated by the dsRNA. This process could be carefully designed to allow pest-specific disruption of proteins critical for life, or essential for reproduction, with no effect on non-target species.
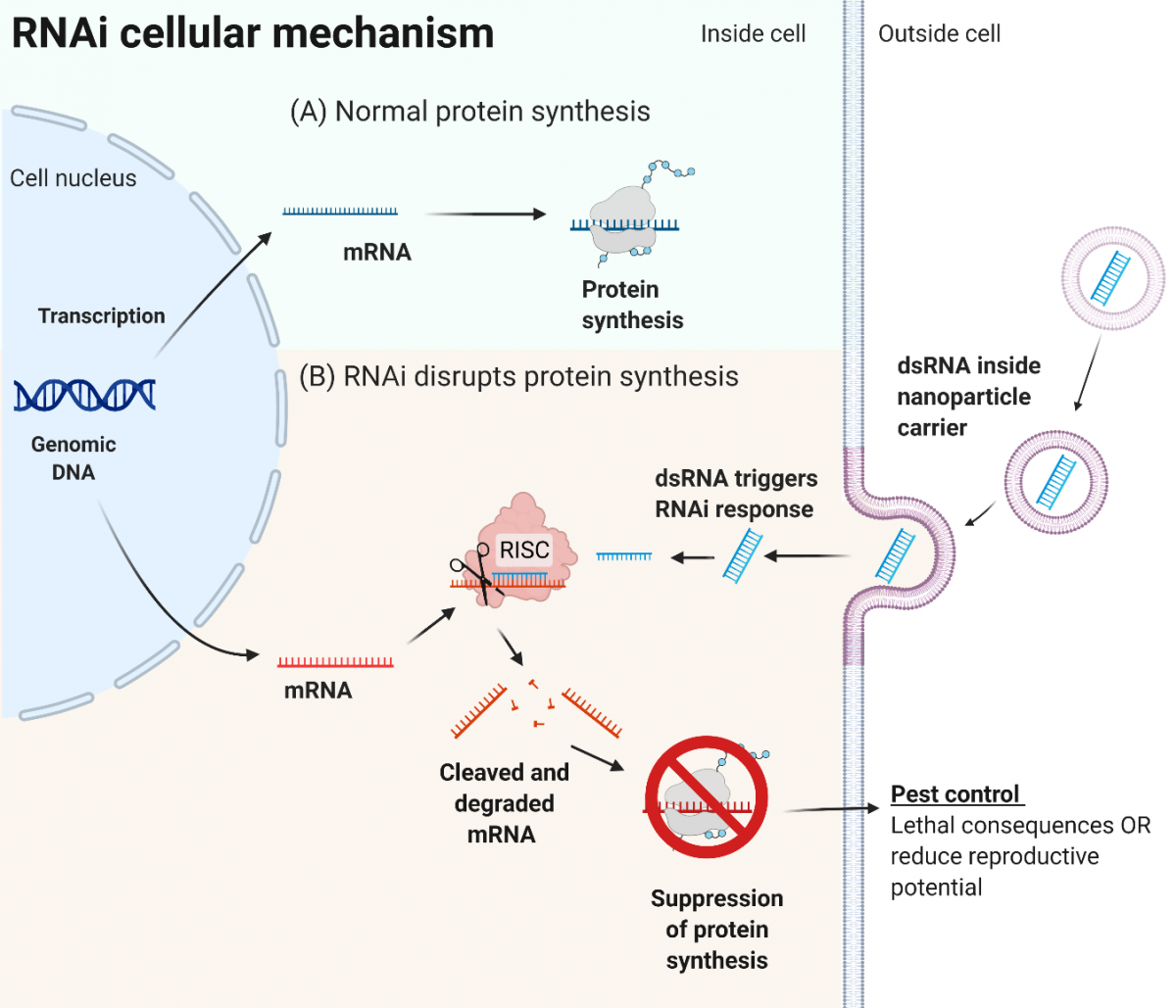
Figure 1. RNAi cellular mechanism. In normal protein synthesis (A), DNA from the genome of the cell is transcribed into messenger RNA (mRNA), which is translated into a protein sequence. The RNAi process (B) is triggered by the presence of dsRNA, which may pass into the cell using a synthetic nanoparticle carrier. Some viruses can also transfer dsRNA into cells. The dsRNA is processed into the RNA-induced Silencing Complex (RISC). This complex cleaves mRNA with a complementary sequence to the dsRNA. This suppresses protein synthesis for the complementary mRNA gene. For pest control, the suppression of protein synthesis would need to have lethal consequences or disrupt reproduction for the pest animal, while considering animal welfare. (Figure created with Biorender.com.)
Although proof-of-concept studies have shown that the RNAi process can be used for the species-specific control of insect pests in an agricultural context, and target-specific RNAi-based human therapeutics are available, such applications are not yet widespread. However, research is rapidly advancing. Combining the methodologies from these evolving research fields could significantly contribute to the development of new RNAi-based vertebrate pest control tools to support Predator Free 2050.
RNAi approaches have a common challenge: delivery of the dsRNA into target cells. For most of the RNAi-based insect pest control in development, dsRNA is produced by genetically modified plants or applied directly to plants for insects to ingest. In human therapeutic applications, the main route of administration for RNAi-based treatment is parenteral (injected) with dsRNA delivery often assisted by carriers, such as nanoparticles.
For cost-effective vertebrate pest control, an oral delivery method would be preferred. However, ingested dsRNA has not yet been shown to affect mammalian health or gene expression. There are still many significant barriers for ingested dsRNA moving from the mammalian gastrointestinal tract to target cells.
The RNAi approach does not affect the genetic material (genome) within the nucleus of the cells. Therefore, RNAi would not involve genetic modification of pest organisms. This differs from other proposed pest control methods such as gene drives, where pests would be genetically modified and released, resulting in multi-generational changes to their reproduction and population suppression. RNAi does not affect the genes passing down from one generation. Therefore, it does not persist in the environment and is more easily controlled. Also, RNAi could potentially be rapidly deployed like traditional toxins rather than being limited by the organism’s generation time. Regardless of the lack of genetic modification of organisms, the use of RNAi technology in Aotearoa environments would need extensive public and regulatory discussion, especially including indigenous voices.
Future research into RNAi would need to overcome these major challenges to unlock the benefits of an RNAi pest control approach (Figure 2). Work would build on the genome sequencing of pest species (possum, stoat, ship rat) that underpins the development of new control technologies. For example, genomes could be used to explore potential target proteins and their sequences. Advances in other areas of RNA-related research could help accelerate the progress of RNAi for pest control. For example, development of nanoparticle biotechnology could inform effective oral delivery methods. Also, the cost of RNA synthesis is likely to decline as RNA-based medicines and RNA insect control methods progress. Our pressing need for new, highly specific vertebrate pest control tools means we should now evaluate the possibility of RNAi for predator control, and its potential effects on Aotearoa ecosystems.
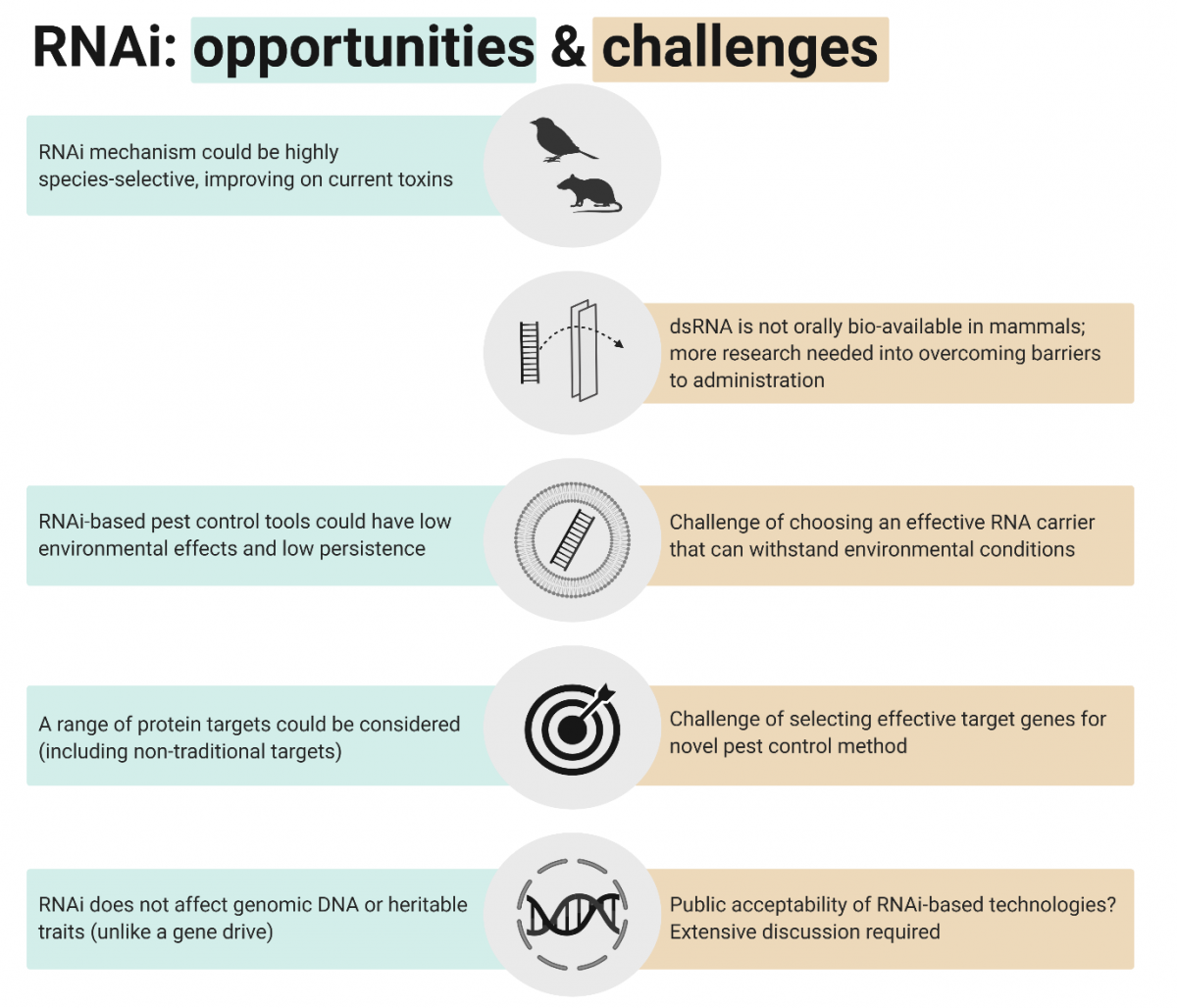
Figure 2. RNAi opportunities and challenges. Potential benefits of an RNAi-based vertebrate pest control approach are shown in teal boxes. Challenges to implementing RNAi as a pest control strategy are shown in orange boxes. (Figure created with Biorender.com.)
Relevant literature:
Dearden, P.K., Gemmell, N.J., Mercier, O.R., Lester, P.J., Scott, M.J., Newcomb, R.D., Buckley, T.R., Jacobs, J.M.E., Goldson, S.G., Penman, D.R. 2018. The potential for the use of gene drives for pest control in New Zealand: a perspective. Journal of the Royal Society of New Zealand 48(4): 225–244. https://doi.org/10.1080/03036758.2017.1385030
Horak, K. E. 2020. RNAi: Applications in Vertebrate Pest Management. Trends in Biotechnology, 38(11): 1200-1202. https://doi.org/10.1016/j.tibtech.2020.05.001
Mercier, O. R., King Hunt, A. and Lester, P. 2019. Novel biotechnologies for eradicating wasps: seeking Māori studies students’ perspectives with Q method. Kōtuitui: New Zealand Journal of Social Sciences Online, 14(1): 136-156. https://doi.org/10.1080/1177083X.2019.1578245
Murphy, E.C., Russell, J.C. Broome, K.G., Ryan, G.J. and Dowding J.E. 2019. Conserving New Zealand’s native fauna: a review of tools being developed for the Predator Free 2050 programme. Journal of Ornithology, 160: 883–892. https://doi.org/10.1007/s10336-019-01643-0
Rodrigues, T. B. and Petrick, J. S. 2020. Safety Considerations for Humans and Other Vertebrates Regarding Agricultural Uses of Externally Applied RNA Molecules. Frontiers in Plant Science, 11: 407. https://doi.org/10.3389/fpls.2020.00407
Vogel, E., Santos, D., Mingels, L., Verdonckt, T. W. and Broeck, J. V. 2018. RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Frontiers in Plant Science, 9: 1912. https://doi.org/10.3389/fphys.2018.01912
Yu, A.-M., Choi, Y. H. and Tu, M.-J. 2020. RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharmacological Reviews, 72(4): 862-898. https://doi.org/10.1124/pr.120.019554
